Organic solid fluorophores regulated by subtle structure modification: color-tunable and aggregation-induced emission†
Abstract
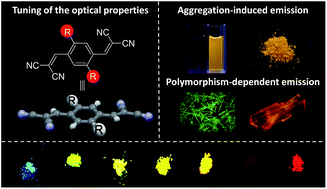
Organic solid fluorophores with a tunable emission color have been widely applied in multiple areas. However, rational design and efficient crafting of these fluorophores from a simple core skeleton is still a formidable challenge because of the undesirable concentration quenching emission effect. Herein, we present the development of two series of organic solid fluorophores based on a backbone of p-bis(2,2-dicyanovinyl)benzene. Notably, the introduction of either non-aromatic or aromatic substituents provides fluorophores with a tunable emission color. Moreover, the fluorophores with aromatic substituents exhibit additional unique optical phenomena, such as aggregation-induced emission, polymorphism-dependent emission, and reversible mechanochromic luminescence.


 Please wait while we load your content...
Please wait while we load your content...