A computational triage approach to the synthesis of novel difluorocyclopentenes and fluorinated cycloheptadienes using thermal rearrangements†
Abstract
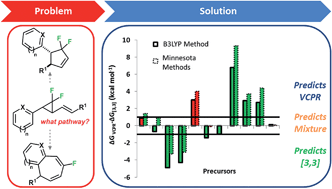
Electronic structure calculations have been used for the effective triage of substituent effects on difluorinated vinylcyclopropane precursors and their ability to undergo vinyl cyclopropane rearrangements (VCPR). Groups which effectively stabilised radicals, specifically heteroarenes, were found to result in the lowest energy barriers. Ten novel precursors were synthesised to test the accuracy of computational predictions; the most reactive species which contained heteroarenes underwent thermal rearrangements at room temperature to afford novel difluorocyclopentenes and fluorinated benzocycloheptadienes through competing VCPR and [3,3]-rearrangement pathways, respectively. More controlled rearrangement of ethyl 3-(1′(2′2′-difluoro-3′-benzo[d][1,3]dioxol-5-yl)cyclopropyl)propenoate (22) allowed these competing pathways to be monitored at the same time and activation energies for both reactions were determined; Ea(VCPR) = (23.4 ± 0.2) kcal mol−1 and Ea([3,3]) = (24.9 ± 0.3) kcal mol−1. Comparing our calculated activation energies with these parameters showed that no single method stood out as the most accurate for predicting barrier heights; (U)M05-2X/6-31+G* methodology remained the best for VCPR but M06-2X/6-31G* was better for the [3,3]-rearrangement. The consistency observed with (U)B3LYP/6-31G* calculations meant that it came closest to a universal method for dealing with these systems. The developed computational design model correctly predicted the observed selectivity of rearrangement pathways for both our system and literature compounds.

 Please wait while we load your content...
Please wait while we load your content...