Palladium-catalyzed regioselective and stereo-invertive ring-opening borylation of 2-arylaziridines with bis(pinacolato)diboron: experimental and computational studies†
Abstract
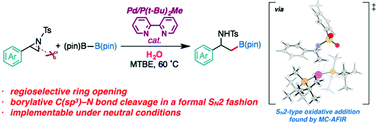
A palladium catalyzed regioselective borylative ring opening reaction of 2-arylaziridines to give β-amino-β-arylethylborates was developed. The reaction reported herein represents the first example of ring-opening borylation of non-vinylic aziridines and direct borylative C(sp3)–N bond cleavage of neutral organic substrates. NMR studies and density functional theory (DFT) calculations suggested that the active intermediate for the reaction is a PdL2 complex [L = P(t-Bu)2Me]. The multi-component artificial force-induced reaction method (MC-AFIR) located the transition states for the regioselectivity-determining aziridine ring opening that proceeds in an SN2 fashion, and explained the selectivity of the reaction. The full catalytic cycle consists of a selectivity-determining aziridine ring opening (oxidative addition), a proton transfer, phosphine ligand dissociation from the catalyst, boron–boron bond cleavage, and reductive elimination. Water is important to the drive the transmetalation step. The calculated overall mechanism and selectivity are consistent with the experimental results.


 Please wait while we load your content...
Please wait while we load your content...