1,3- and 1,4-Benzdiyne equivalents for regioselective synthesis of polycyclic heterocycles†
Abstract
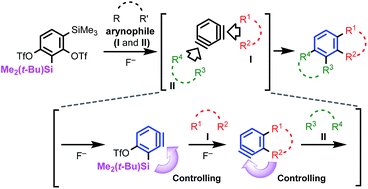
We have devised a novel 1,3-benzdiyne equivalent, capable of quadruple functionalization by sequential benzyne generation and reaction with arynophiles. The key features of this method include the chemoselective generation of two triple bonds in a single benzene ring under fluoride-mediated mild conditions, and the regiocontrol of each benzyne reaction by the substituent next to the triple bond. This method produced various benzo-fused heteroaromatic compounds via reactions with arynophiles, such as furans, azides, and diazo compounds. A validation of the method is given in the convergent synthesis of the antipsychotic drug risperidone. A similar strategy has also been applied to a 1,4-benzdiyne equivalent to construct linearly benzo-fused heteroaromatics.

 Please wait while we load your content...
Please wait while we load your content...