A versatile glycosylation strategy via Au(iii) catalyzed activation of thioglycoside donors†
Abstract
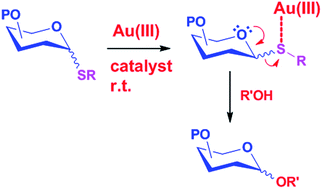
Among various methods of chemical glycosylations, glycosylation by activation of thioglycoside donors using a thiophilic promoter is an important strategy. Many promoters have been developed for the activation of thioglycosides. However, incompatibility with substrates having alkenes and the requirement of a stoichiometric amount of promoters, co-promoters and extreme temperatures are some of the limitations. We have developed an efficient methodology for glycosylation via the activation of thioglycoside donors using a catalytic amount of AuCl3 and without any co-promoter. The reaction is very fast, high-yielding and very facile at room temperature. The versatility of this method is evident from the facile glycosylation with both armed and disarmed donors and sterically demanding substrates (acceptors/donors) at ambient conditions, from the stability of the common protecting groups, and from the compatibility of alkene-containing substrates during the reaction.


 Please wait while we load your content...
Please wait while we load your content...