Methane activation by gold-doped titanium oxide cluster anions with closed-shell electronic structures†
Abstract
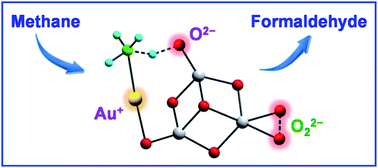
The reactivity of closed-shell gas phase cluster anions AuTi3O7− and AuTi3O8− with methane under thermal collision conditions was studied by mass spectrometric experiments and quantum chemical calculations. Methane activation was observed with the formation of AuCH3 in both cases, while the formation of formaldehyde was also identified in the reaction system of AuTi3O8−. The cooperative effect of the separated Au+ and O2− ions on the clusters induces the cleavage of the first C–H bond of methane. Further activation of the second C–H bond by a peroxide ion O22− leads to the formation of formaldehyde. This study shows that closed-shell species on metal oxides can be reactive enough to facilitate thermal H–CH3 bond cleavage and the subsequent conversion.

 Please wait while we load your content...
Please wait while we load your content...