Enhancement of dye regeneration kinetics in dichromophoric porphyrin–carbazole triphenylamine dyes influenced by more exposed radical cation orbitals†
Abstract
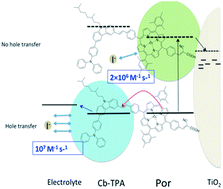
Reduction kinetics of oxidized dyes absorbed on semiconductor surfaces and immersed in redox active electrolytes has been mainly modeled based on the free energy difference between the oxidation potential of the dye and the redox potential of the electrolyte. Only a few mechanisms have been demonstrated to enhance the kinetics by other means. In this work, the rate constant of the reduction of oxidized porphyrin dye is enhanced by attaching non-conjugated carbazole triphenylamine moiety using iodine/triiodide and tris(2,2′-bispyridinium)cobalt II/III electrolytes. These results are obtained using transient absorption spectroscopy by selectively probing the regeneration kinetics at the porphyrin radical cation and the carbazole triphenylamine radical cation absorption wavelengths. The enhancement in the reduction kinetics is not attributed to changes in the driving force, but to the more exposed dye cation radical orbitals of the dichromophoric dye. The results are important for the development of high efficiency photo-electrochemical devices with minimalized energy loss at electron transfer interfaces.
- This article is part of the themed collection: Global Energy Challenges: Solar Energy


 Please wait while we load your content...
Please wait while we load your content...