The involvement of the trisulfur radical anion in electron-catalyzed sulfur insertion reactions: facile synthesis of benzothiazine derivatives under transition metal-free conditions†
Abstract
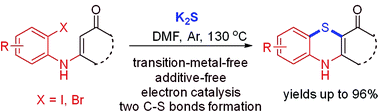
An efficient and practical synthesis of benzothiazine by K2S initiated sulfur insertion reaction with enaminones via electron catalysis is developed. This protocol provides a new, environment-friendly and simple strategy to construct benzothiazine derivatives via formation of two C–S bonds under transition metal-free, additive-free and oxidant-free conditions. K2S not only provides the sulfur insertion source, but also ignites the reaction through the formation of a trisulfur radical anion and electrons in DMF.
- This article is part of the themed collection: SU 120: Celebrating 120 Years of Soochow University

 Please wait while we load your content...
Please wait while we load your content...