Chemoselective dehydrogenative esterification of aldehydes and alcohols with a dimeric rhodium(ii) catalyst†
Abstract
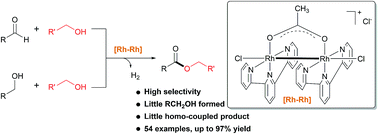
Dehydrogenative cross-coupling of aldehydes with alcohols as well as dehydrogentive cross-coupling of primary alcohols to produce esters have been developed using a Rh-terpyridine catalyst. The catalyst demonstrates broad substrate scope and good functional group tolerance, affording esters highly selectively. The high chemoselectivity of the catalyst stems from its preference for dehydrogenation of benzylic alcohols over aliphatic ones. Preliminary mechanistic studies suggest that the active catalyst is a dimeric Rh(II) species, operating via a mechanism involving metal–base–metal cooperativity.


 Please wait while we load your content...
Please wait while we load your content...