“Inverse” thermoresponse: heat-induced double-helix formation of an ethynylhelicene oligomer with tri(ethylene glycol) termini†
Abstract
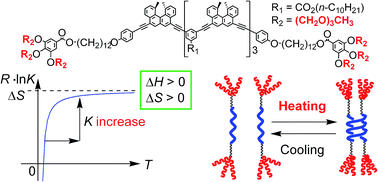
An ethynylhelicene oligomer [(M)-D-4]-C12-TEG with six tri(ethylene glycol) (TEG) groups at the termini was synthesized, and double-helix formation was studied using CD, UV-Vis, vapor pressure osmometry, dynamic light scattering, and 1H NMR. [(M)-D-4]-C12-TEG reversibly changed its structure between a double helix and a random coil in response to heating and cooling in aromatic solvents, non-aromatic polar organic solvents, and aqueous solvent mixtures of acetone/water/triethylamine. Notably, [(M)-D-4]-C12-TEG in acetone/water/triethylamine (1/2/1) formed a double helix upon heating and disaggregated into random coils upon cooling. The double helix/random coil ratio sharply changed in response to temperature changes. This is an unprecedented “inverse” thermoresponse, which is opposite to the “ordinary” thermoresponse in molecular dimeric aggregate formation. This phenomenon was explained by the dehydration of the terminal TEG groups and the formation of condensed triethylamine domains upon heating.

 Please wait while we load your content...
Please wait while we load your content...