Comment on “A quantitative definition of hypervalency” by M. C. Durrant, Chem. Sci., 2015, 6, 6614
Abstract
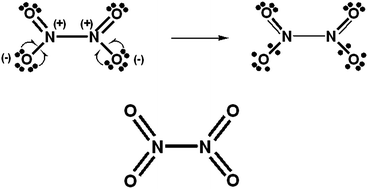
Consideration is given to (electronically) hypervalent increased-valence structures, which possess 2c–1e bonds, fractional 2c–2e bonds, and usually normal 2c–2e bonds. For singlet-spin electron-rich systems, increased-valence structures, with Heitler–London 2c–2e bond wavefunctions, are equivalent to resonance between non-hypervalent Kekulé and Dewar (or singlet diradical) type Lewis structures. Dewar structures are not considered in the Chem. Sci. 2015, 6, 6614 Edge article on hypervalency. Using one-electron delocalizations from lone-pair atomic orbitals into separate bonding molecular orbitals, increased-valence structures for PCl5, O3, SO42−, NO3−, N2O4 and SN2 reactions are derived from the Edge-article's Kekulé-type Lewis structures, and compared with the Edge article's hypervalent structures with 2c–2e bonds. It is also shown that Durrant's method to determine the γ parameter for XAY-type systems that possess a symmetrical 3c–4e bonding unit is related to the A-atom charge density.

 Please wait while we load your content...
Please wait while we load your content...