Extremely condensing triplet states of DPEPO-type hosts through constitutional isomerization for high-efficiency deep-blue thermally activated delayed fluorescence diodes†
Abstract
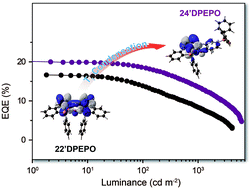
The similarity of thermally activated delayed fluorescence (TADF) dyes and their hosts as pure organic molecules makes hosts predominant in intermolecular interactions and crucial to exciton harvesting and utilization in TADF diodes. DPEPO is the most popular high-energy-gap blue TADF host with steric ortho-substituted diphenylphosphine oxide (DPPO) groups for intermolecular interaction suppression, but suffers from serious efficiency roll-off due to its weak electroactivity. On the contrary, para-substituted DPPO with small steric hindrance is superior in intramolecular electronic coupling. In this work, four constitutional isomers of DPEPO are constructed as diphenylether (DPE) with two diphenylphosphine oxide (DPPO) groups substituted at either the 2 or 4 position, namely 22′DPEPO (viz.DPEPO), 24DPEPO, 24′DPEPO and 44′DPEPO, respectively. On the basis of separation configuration, the steric effect and electroactivity of ortho- and para-substituted DPPOs are successfully integrated in 24′DPEPO, accompanied by remarkably reduced intermolecular interactions due to its unsymmetrical configuration. Compared to its congeners, 24′DPEPO has a rigid structure and locally excited states similar to 22′DPEPO for interaction suppression and improved charge mobility comparable to 44′DPEPO for charge flux balance. Significantly, by virtue of the predominant orientation effect of ortho-DPPO on the T1 location, its T1 state is extremely condensed onto a single phenyl, protected from intermolecular interactions by its remaining five phenyls at its maximum extent. Consequently, 24′DPEPO endowed its DMAC-DPS-based deep-blue devices with state-of-the-art performance, including high color purity with chromaticity coordinates of (0.16, 0.17), external quantum efficiency (EQE) beyond 20% and EQE roll-off as low as 32% at 1000 cd m−2. It is shown that the device performance of 24′DPEPO was far beyond simple integration of those of 22′DPEPO and 44′DPEPO, verifying the significance of host optimization.

 Please wait while we load your content...
Please wait while we load your content...