The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation†
Abstract
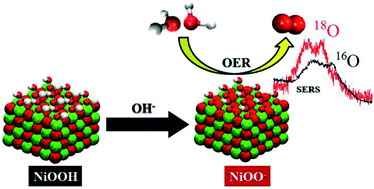
Nickel oxyhydroxide (NiOOH) is extensively used for energy storage and it is a very promising catalyst for the oxygen evolution reaction (OER). However, the processes occurring on the NiOOH surface during charge accumulation and OER are not well understood. This work presents an in situ Surface Enhanced Raman Spectroscopy (SERS) study of the pH dependent interfacial changes of the NiOOH catalyst under the working conditions used for OER. We demonstrate the important effect of the electrolyte pH on the degree of surface deprotonation of NiOOH, which crucially affects its OER activity. Our results show that the deprotonation of NiOOH produces negatively charged (or proton-deficient) surface species, which are responsible for the enhanced OER activity of NiOOH in highly alkaline pH. Moreover, we provide spectroscopic evidence obtained in an 18O-labeled electrolyte that allows us to assign this surface species to a superoxo-type species (Ni–OO−). Furthermore, we propose a mechanism for the OER on NiOOH which is consistent with the observed pH-sensitivity, and that also explains why NiOOH is not a suitable catalyst for applications in neutral or moderately alkaline pH (in the range 7–11), apart from the lower stability of the catalyst under these conditions.
- This article is part of the themed collections: Global Energy Challenges: Electrochemical Energy and Global Energy Challenges: Energy Applications


 Please wait while we load your content...
Please wait while we load your content...