Origins of unique gold-catalysed chemo- and site-selective C–H functionalization of phenols with diazo compounds†
Abstract
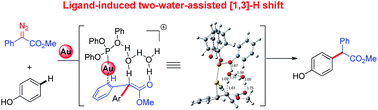
In past decade, gold revealed more and more unique properties in carbene chemistry. It was disclosed in our recent communication (J. Am. Chem. Soc. 2014, 136, 6904) that gold carbenes have unprecedented chemo- and site-selectivity and ligand effect toward the functionalization of C–H bonds in phenols. In this full article, we report a comprehensively combined theoretical and experimental study on the mechanism of the insertion of gold carbenes into C–H and O–H bonds in phenol. It significantly revealed that the ligands have an important effect on C–H insertion and the reaction proceeds through a pathway involving the formation of an enolate-like intermediate. Moreover, two water molecules serving as a proton shuttle are believed to be the key issue for achieving chemoselective C–H functionalization, which is strongly supported by the DFT calculations and control experiments. It is the first time that a clear explanation is given about the prominent catalysis of gold carbenes toward C–H functionalization based on a theoretical and experimental study.

 Please wait while we load your content...
Please wait while we load your content...