Directing group assisted meta-hydroxylation by C–H activation†
Abstract
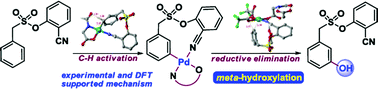
meta-Hydroxylated cores are ubiquitous in natural products. Herein, we disclose the first template assisted meta-hydroxylation reaction. Experimental and in silico studies helped us to gain valuable mechanistic insights, including the role of the hexafluoroisopropanol (HFIP) solvent, during C–H hydroxylation. The reactive intermediates, prior to the C–H activation, have been detected by spectroscopic techniques. Additionally, the C–O bond formation has been extended to meta-acetoxylation. The preparation of a phase II quinone reductase activity inducer and a resveratrol precursor illustrated the synthetic significance of the present strategy.
- This article is part of the themed collection: ISACS19: Challenges in Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...