The fabrication of a supra-amphiphile for dissipative self-assembly†
Abstract
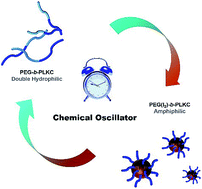
Dissipative self-assembly is a challenging but attractive field of supramolecular science, because it generally concerns complex systems but is more close to the self-assembly of living bodies. In this article, we realized dissipative self-assembly by coupling a supra-amphiphile with a chemical oscillator. The supra-amphiphile was fabricated with iodine and a double hydrophilic block copolymer containing PEG segments, as the non-covalent interaction between PEG and iodine could turn PEG hydrophobic, leading to the formation of the supra-amphiphile. The self-assembly and disassembly of the supra-amphiphile could be controlled by varying the concentration of iodine. Therefore, the dissipative self-assembly of the supra-amphiphile was realized when it was coupled with the IO3−–NH3OH+–OH− chemical oscillator, which was able to produce iodine periodically. Meanwhile, the kinetic data of the self-assembly and disassembly of the supra-amphiphile could be estimated by the theoretical simulation of the chemical oscillations. This line of research promotes the self-assembly of supra-amphiphiles one step forward from thermodynamic statics to a dissipative system, and also suggests a new strategy to investigate the kinetics of stimuli-responsive molecular self-assembly.
- This article is part of the themed collection: ISACS21: Challenges in Nanoscience

 Please wait while we load your content...
Please wait while we load your content...