Recognition of extended linear and cyclised polyketide mimics by a type II acyl carrier protein†
Abstract
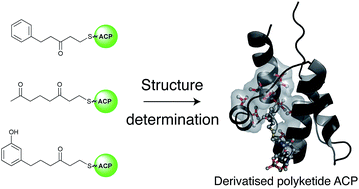
Polyketides are secondary metabolites which display both valuable pharmaceutical and agrochemical properties. Biosynthesis is performed by polyketide synthases (PKSs), and the acyl carrier protein (ACP), a small acidic protein, that transports the growing polyketide chain and is essential for activity. Here we report the synthesis of two aromatic probes and a linear octaketide mimic that have been tethered to actinorhodin ACP. These experiments were aimed at probing the ACP's capacity to sequester a non-polar versus a phenolic aromatic ring (that more closely mimics a polyketide intermediate) as well as investigations with extended polyketide chain surrogates. The binding of these mimics has been assessed using high-resolution solution NMR studies and high-resolution structure determination. These results reveal that surprisingly a PKS ACP is able to bind and sequester a bulky non-polar substrate containing an aromatic ring in a fatty acid type binding mode, but the introduction of even a small degree of polarity favours a markedly different association at a surface site that is distinct from that employed by fatty acid ACPs.


 Please wait while we load your content...
Please wait while we load your content...