Double conjugation strategy to incorporate lipid adjuvants into multiantigenic vaccines†
Abstract
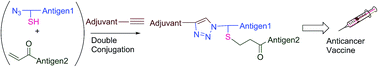
Conjugation of multiple peptides by their N-termini is a promising technique to produce branched multiantigenic vaccines. We established a double conjugation strategy that combines a mercapto-acryloyl Michael addition and a copper-catalysed alkyne-azide 1,3-dipolar cycloaddition (CuAAC) reaction to synthesise self-adjuvanting branched multiantigenic vaccine candidates. These vaccine candidates aim to treat cervical cancer and include two HPV-16 derived epitopes and a novel self-adjuvanting moiety. This is the first report of mercapto-acryloyl conjugation applied to the hetero conjugation of two unprotected peptides by their N-termini followed by a CuAAC reaction to conjugate a novel synthetic lipoalkyne self-adjuvanting moiety. In vivo experiments showed that the most promising vaccine candidate completely eradicated tumours in 46% of the mice (6 out of 13 mice).

 Please wait while we load your content...
Please wait while we load your content...