Systematic study of the dynamics and half-lives of newly synthesized proteins in human cells†
Abstract
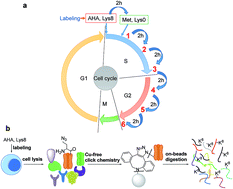
Protein dynamics are essential in regulating nearly every cellular event, and aberrant proteostasis is the source of many diseases. It is extraordinarily difficult to globally study protein dynamics and accurately measure their half-lives. Here we have developed a chemical proteomics method integrating protein labeling, click chemistry and multiplexed proteomics, which overcomes current challenges with existing methods. Labeling with both azidohomoalanine (AHA) and heavy lysine allows us to selectively enrich newly synthesized proteins, clearly distinguish them from existing proteins, and reduce the impact of heavy amino acid recycling. Moreover, multiplexed proteomics enables us to quantify proteins at multiple time points simultaneously, thus increasing the accuracy of measuring protein abundance changes and their half-lives. Systematic investigation of newly synthesized protein dynamics will provide insight into proteostasis and the molecular mechanisms of disease.

 Please wait while we load your content...
Please wait while we load your content...