Nickel-catalyzed arylation of heteroaryl-containing diarylmethanes: exceptional reactivity of the Ni(NIXANTPHOS)-based catalyst†
Abstract
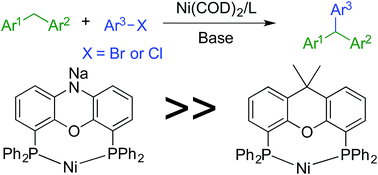
Nickel(0)-catalyzed cross-coupling of heteroaryl-containing diarylmethanes with both aryl bromides and chlorides has been achieved. The success of this reaction relies on the introduction of a unique nickel/NIXANTPHOS-based catalyst system, which provides a direct route to triarylmethanes from heteroaryl-containing diarylmethanes. Reactivity studies indicate the Ni(NIXANTPHOS)-based catalyst exhibits enhanced reactivity over XANTPHOS derivatives and other Ni(phosphine)-based catalysts in the reactions examined.

 Please wait while we load your content...
Please wait while we load your content...