Enantiodivergent asymmetric catalysis with the tropos BIPHEP ligand and a proline derivative as chiral selector†
Abstract
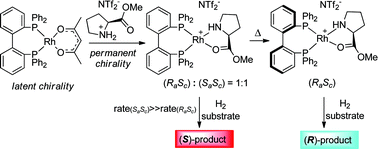
A catalytic system based on the tropos ligand BIPHEP and (S)-proline methyl ester as chiral selector was studied for Rh-catalysed asymmetric catalysis. By careful control of the catalyst preformation conditions, the enantioselectivity could be completely reversed in asymmetric hydrogenation of prochiral olefins maintaining the same absolute level in favorable cases. The enantiodivergent asymmetric catalysis could be rationalised by the interplay of the dynamic chirality (tropos) of the phosphine ligand and the coordination of the proline selector. Treating a suitable Rh-BIPHEP precursor with the (Sc)-proline-based ionic liquid led to an equimolar mixture of (RaSc)- and (SaSc)-diastereomers that is kinetically stable at 0 °C. At higher temperature, an irreversible diastereomerisation process was observed resulting in the diastereomerically pure (RaSc)-complex [Rh{(Ra)-BIPHEP}{(Sc)-ProlOMe}]. Whereas the use of the pure (RaSc)-complex led to 51% ee (R) in the hydrogenation of methyl 2-acetamidoacrylate, the S-product was formed with almost identical enantioselectivity when the (RaSc)/(SaSc)-mixture was applied under identical conditions. This inversion was associated with the relative stability of the diastereomers in the equilibria forming the catalytically active substrate complex. The possibility to use this different reactivity to control the direction of enantioselectivity was demonstrated for the hydrogenation of different substrates whereby ee's of up to 80% could be achieved. Moreover, the (RaSc)-complex led to high enantioselectivities of up 86% ee in the asymmetric hydroboration of styrene, approaching the performance of the atropos BINAP ligand for this reaction.

 Please wait while we load your content...
Please wait while we load your content...