Catalytic activation of a single C–F bond in trifluoromethyl arenes†
Abstract
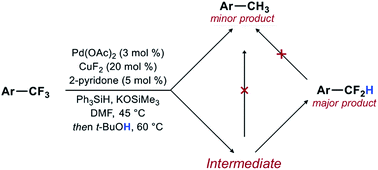
Synthetic methods for the direct transformation of ArCF3 to ArCF2R would enable efficient diversification of trifluoromethyl arenes and would be of great utility in medicinal chemistry. Unfortunately, the development of such methods has been hampered by the fundamental properties of C–F bonds, which are exceptionally strong and become stronger with increased fluorination of the carbon atom. Here, we describe a method for the catalytic reduction of ArCF3 to ArCF2H through a highly selective activation of a single C–F bond. Mechanistic studies reveal separate reaction pathways for the formation of ArCF2H and ArCH3 products and point to the formation of an unexpected intermediate as the source of the unusual selectivity for the mono-reduction.

 Please wait while we load your content...
Please wait while we load your content...