Cooperative duplex formation by synthetic H-bonding oligomers†
Abstract
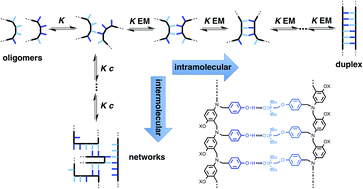
A series of flexible oligomers equipped with phenol H-bond donors and phosphine oxide H-bond acceptors have been synthesised using reductive amination chemistry. H-bonding interactions between complementary oligomers leads to the formation of double-stranded complexes which were characterised using NMR titrations and thermal denaturation experiments. The stability of the duplex increases by one order of magnitude for every H-bonding group added to the chain. Similarly, the enthalpy change for duplex assembly and the melting temperature for duplex denaturation both increase with increasing chain length. These observations indicate that H-bond formation along the oligomers is cooperative despite the flexible backbone, and the effective molarity for intramolecular H-bond formation (14 mM) is sufficient to propagate the formation of longer duplexes using this approach. The product K EM, which is used to quantify chelate cooperativity is 5, which means that each H-bond is more than 80% populated in the assembled duplex. The modular design of these oligomers represents a general strategy for the design of synthetic information molecules that could potentially encode and replicate chemical information in the same way as nucleic acids.

 Please wait while we load your content...
Please wait while we load your content...