Cationic porphyrins are tunable gatekeepers of the 20S proteasome†
Abstract
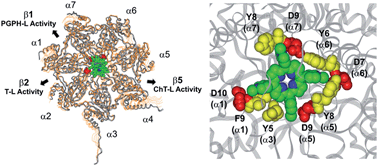
The 20S proteasome is a barrel-shaped enzymatic assembly playing a critical role in proteome maintenance. Access of proteasome substrates to the catalytic chamber is finely regulated through gating mechanisms which involve aromatic and negatively charged residues located at the N-terminal tails of α subunits. However, despite the importance of gates in regulating proteasome function, up to now very few molecules have been shown to interfere with the equilibrium by which the catalytic channel exchanges between the open and closed states. In this light, and inspired by previous results evidencing the antiproteasome potential of cationic porphyrins, here we combine experimental (enzyme kinetics, UV stopped flow and NMR) and computational (bioinformatic analysis and docking studies) approaches to inspect proteasome inhibition by meso-tetrakis(4-N-methylpyridyl)-porphyrin (H2T4) and its two ortho- and meta-isomers. We show that in a first, fast binding event H2T4 accommodates in a pocket made of negatively charged and aromatic residues present in α1 (Asp10, Phe9), α3 (Tyr5), α5 (Asp9, Tyr8), α6 (Asp7, Tyr6) and α7 (Asp9, Tyr8) subunits thereby stabilizing the closed conformation. A second, slower binding mode involves interaction with the grooves which separate the α- from the β-rings. Of note, the proteasome inhibition by ortho- and meta-H2T4 decreases significantly if compared to the parent compound, thus underscoring the role played by spatial distribution of the four peripheral positive charges in regulating proteasome–ligand interactions. We think that our results may pave the way to further studies aimed at rationalizing the molecular basis of novel, and more sophisticated, proteasome regulatory mechanisms.


 Please wait while we load your content...
Please wait while we load your content...