Synthesis, structure, and reactions of a copper–sulfido cluster comprised of the parent Cu2S unit: {(NHC)Cu}2(μ-S)†
Abstract
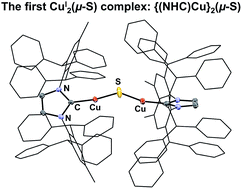
The synthesis of the first CuI2(μ-S) complex, {(IPr*)Cu}2(μ-S) (IPr* = 1,3-bis(2,6-(diphenylmethyl)-4-methylphenyl)imidazol-2-ylidene; 1), has been accomplished via three synthetic routes: (1) salt metathesis between (IPr*)CuCl and Na2S; (2) silyl-deprotection reaction between (IPr*)Cu(SSiMe3) and (IPr*)CuF; and (3) acid–base reaction between (IPr*)Cu(SH) and (IPr*)Cu(OtBu). The X-ray crystal structure of 1 exhibits two two-coordinate copper centers connected by a bent Cu–S–Cu linkage. Application of these synthetic routes to analogous precursors containing the sterically smaller ligand IPr (1,3-bis(2,6-di-isopropylphenyl)imidazol-2-ylidene), in place of IPr*, resulted in the formation of a transient product proposed as {(IPr)Cu}2(μ-S) (2), which decomposes quickly in solution. The instability of 2 probably results from the insufficient steric protection provided by IPr ligands to the unsaturated Cu2(μ-S) core; in contrast, 1 is stable both in solution and solid state for weeks. The nucleophilic sulfido ligand in 1 reacts with haloalkyl electrophiles (benzyl halides and dibromoalkanes) with formation of C–S bonds, affording (IPr*)Cu(SCH2Ph) and cyclic thioethers, respectively.

 Please wait while we load your content...
Please wait while we load your content...