N6-Methyladenine hinders RNA- and DNA-directed DNA synthesis: application in human rRNA methylation analysis of clinical specimens†
Abstract
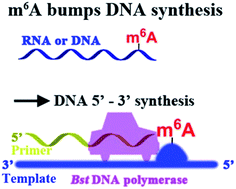
N 6-Methyladenine (m6A) is the most abundant internal modification on mammalian mRNA. Very recently, m6A has been reported as a potentially important ‘epigenetic’ mark in eukaryotes. Until now, site-specific detection of m6A is technically very challenging. Here, we first reveal that m6A significantly hinders DNA- and RNA-directed DNA synthesis. Systematic investigations of 5′-triphosphates of a variety of 5-substituted 2′-deoxyuridine analogs in primer extension have been performed. In the current study, a quantitative analysis of m6A in the RNA or DNA context has been achieved, using Bst DNA polymerase catalyzed primer extension. Molecular dynamics study predicted that m6A in template tends to enter into and be restrained in the MGR region of Bst DNA polymerase, reducing conformational flexibility of the DNA backbone. More importantly, a site-specific determination of m6A in human ribosomal RNA (rRNA) with high accuracy has been afforded. Through a cumulative analysis of methylation alterations, we first reveal that significantly cancer-related changes in human rRNA methylation were present in patients with hepatocellular carcinoma.

 Please wait while we load your content...
Please wait while we load your content...