Isolation of +2 rare earth metal ions with three anionic carbocyclic rings: bimetallic bis(cyclopentadienyl) reduced arene complexes of La2+ and Ce2+ are four electron reductants†
Abstract
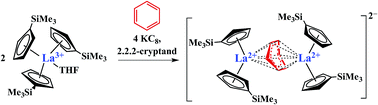
A new option for stabilizing unusual Ln2+ ions has been identified in the reaction of Cp′3Ln, 1-Ln (Ln = La, Ce; Cp′ = C5H4SiMe3), with potassium graphite (KC8) in benzene in the presence of 2.2.2-cryptand. This generates [K(2.2.2-cryptand)]2[(Cp′2Ln)2(μ-η6:η6-C6H6)], 2-Ln, complexes that contain La and Ce in the formal +2 oxidation state. These complexes expand the range of coordination environments known for these ions beyond the previously established examples, (Cp′′3Ln)1− and (Cp′3Ln)1− (Cp′′ = C5H3(SiMe3)2-1,3), and generalize the viability of using three anionic carbocyclic rings to stabilize highly reactive Ln2+ ions. In 2-Ln, a non-planar bridging (C6H6)2− ligand shared between two metals takes the place of a cyclopentadienyl ligand in (Cp′3Ln)1−. The intensely colored (ε = ∼8000 M−1 cm−1) 2-Ln complexes react as four electron reductants with two equiv. of naphthalene to produce two equiv. of the reduced naphthalenide complex, [K(2.2.2-cryptand)][Cp′2Ln(η4-C10H8)].

 Please wait while we load your content...
Please wait while we load your content...