Magnesium-catalyzed hydrosilylation of α,β-unsaturated esters†
Abstract
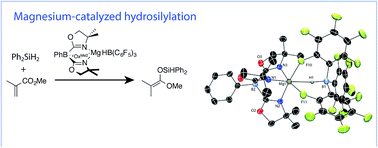
ToMMgHB(C6F5)3 (1, ToM = tris(4,4-dimethyl-2-oxazolinyl)phenylborate) catalyzes the 1,4-hydrosilylation of α,β-unsaturated esters. This magnesium hydridoborate compound is synthesized by the reaction of ToMMgMe, PhSiH3, and B(C6F5)3. Unlike the transient ToMMgH formed from the reaction of ToMMgMe and PhSiH3, the borate adduct 1 persists in solution and in the solid state. Crystallographic characterization reveals tripodal coordination of the HB(C6F5)3 moiety to the six-coordinate magnesium center with a ∠Mg–H–B of 141(3)°. The pathway for formation of 1 is proposed to involve the reaction of ToMMgMe and a PhSiH3/B(C6F5)3 adduct because the other possible intermediates, ToMMgH and ToMMgMeB(C6F5)3, react to give an intractable black solid and ToMMgC6F5, respectively. Under catalytic conditions, silyl ketene acetals are isolated in high yield from the addition of hydrosilanes to α,β-unsaturated esters with 1 as the catalyst.

 Please wait while we load your content...
Please wait while we load your content...