Stereoelectronic source of the anomalous stability of bis-peroxides†
Abstract
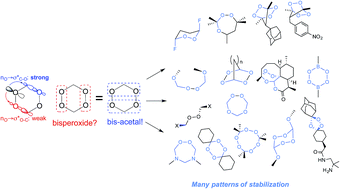
The unusual stability of bis- and tris-peroxides contradicts the conventional wisdom – some of them can melt without decomposition at temperatures exceeding 100 °C. In this work, we disclose a stabilizing stereoelectronic effect that two peroxide groups can exert on each other. This stabilization originates from strong anomeric nO → σ*CO interactions that are absent in mono-peroxides but reintroduced in molecules where two peroxide moieties are separated by a CH2 group. Furthermore, such effects can be induced by other σ-acceptors and amplified by structural constraints imposed by cyclic and bicyclic frameworks.

 Please wait while we load your content...
Please wait while we load your content...