Catalytic arylsulfonyl radical-triggered 1,5-enyne-bicyclizations and hydrosulfonylation of α,β-conjugates†
Abstract
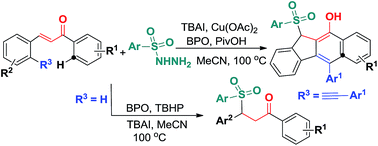
A catalytic bicyclization reaction of 1,5-enynes anchored by α,β-conjugates with arylsulfonyl radicals generated in situ from sulfonyl hydrazides has been established using TBAI (20 mol%) and Cu(OAc)2 (5 mol%) as co-catalysts under convenient conditions. In addition, the use of benzoyl peroxide (BPO) as the oxidant and pivalic acid (PivOH) as an additive was proven to be necessary for this reaction. The reactions occurred through 5-exo-dig/6-endo-trig bicyclizations and homolytic aromatic substitution (HAS) cascade mechanisms to give benzo[b]fluorens regioselectively. A similar catalytic process was developed for the synthesis of γ-ketosulfones. These reactions feature readily accessible starting materials and simple one-pot operation.

 Please wait while we load your content...
Please wait while we load your content...