Rh-catalyzed reagent-free ring expansion of cyclobutenones and benzocyclobutenones†
Abstract
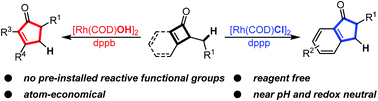
Here we report a reagent-free rhodium-catalyzed ring-expansion reaction via C–C cleavage of cyclobutenones. A variety of poly-substituted cyclopentenones and 1-indanones can be synthesized from simple cyclobutenones and benzocyclobutenones. The reaction condition is near pH neutral without additional oxidants or reductants. The potential for developing a dynamic kinetic asymmetric transformation of this reaction has also been demonstrated. Further study supports the proposed pathway involving Rh-insertion into the cyclobutenone C–C bond, followed by β-hydrogen elimination, olefin insertion and reductive elimination.

 Please wait while we load your content...
Please wait while we load your content...