Mechanistic insights into cobalt(ii/iii)-catalyzed C–H oxidation: a combined theoretical and experimental study†
Abstract
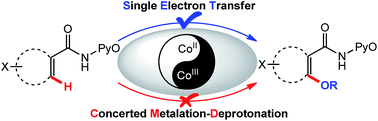
Cobalt-mediated C–H functionalization has been the subject of extensive interest in synthetic chemistry, but the mechanisms of many of these reactions (such as the cobalt-catalyzed C–H oxidation) are poorly understood. In this paper, possible mechanisms including single electron transfer (SET) and the concerted metalation–deprotonation (CMD) pathways of the CoII/CoIII-catalyzed alkoxylation of C(sp2)–H bonds have been investigated for the first time using the DFT method. CoII(OAc)2 has been employed as an efficient catalyst in our previous experimental study, but the calculated results unexpectedly indicated that the intermolecular SET pathway with CoIII as the actual catalyst might be the most favorable pathway. To support this theoretical prediction, we have explored a series of Cp*CoIII(CO)I2 catalyzed C(sp2)–H bond alkoxylations, extending the application of cobalt-catalyzed functionalization of C–H bonds. Furthermore, kinetic isotope effect (KIE) data, electron paramagnetic resonance (EPR) data, and TEMPO inhibition experiments also support the SET mechanism in both the Co-catalyzed alkoxylation reactions. Thus, this work should support an understanding of the possible mechanisms of the CoII/CoIII-catalyzed C(sp2)–H functionalization, and also provide an example of the rational design of novel catalytic reactions guided by theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...