Palladium-catalyzed direct β-arylation of ketones with diaryliodonium salts: a stoichiometric heavy metal-free and user-friendly approach†
Abstract
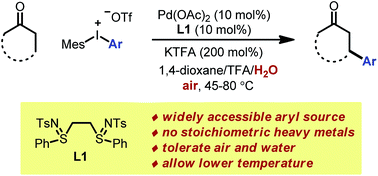
We herein report a new protocol for the Pd-catalyzed β-arylation of ketones without stoichiometric heavy metals. Widely accessible diaryliodonium salts are used as both the oxidant and aryl source. This tandem redox catalysis merges ketone dehydrogenation and conjugate addition without an additional oxidant or reductant. This transformation features the use of a unique bis-N-tosylsulfilimine ligand and the combination of potassium trifluoroacetate/trifluoroacetic acid to maintain an appropriate acidity of the reaction medium. The reaction tolerates both air and moisture, and shows a broad substrate scope. Kinetics studies, along with filtration and poisoning tests, support the involvement of palladium nanoparticles in the catalysis.

 Please wait while we load your content...
Please wait while we load your content...