A nitrogen-base catalyzed generation of organotin(ii) hydride from an organotin trihydride under reductive dihydrogen elimination†
Abstract
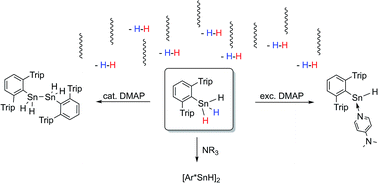
Since their first description over a decade ago, organotin(II) hydrides have been an iconic class of compounds in molecular main group chemistry. Among other approaches they have been accessed from the hydrogenation of distannynes. We herein report their accessibility from the other direction by dehydrogenation of organotin trihydride. On reacting pyridine and amine bases with the bulky substituted organotin trihydride Ar*SnH3 (Ar* = 2,6-trip2(C6H3)–, trip = 2,4,6-triisopropylphenyl) hydrogen evolution was observed. In case of catalytic amounts of base the dehydro-coupling product diorganodistannane Ar*H2SnSnH2Ar* was obtained quantitatively whilst for excessive amounts (>4 eq.) the monomeric base adduct to known Ar*SnH was obtained almost exclusively. The base adducts were found to be remarkably thermally robust. They readily react with polar fulvenic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-bonds in hydro-stannylenylation reactions. The resulting half-sandwich complex Ar*SnCp* was structurally characterized. Moreover, on application of less nucleophilic amine bases, the uncoordinated, in solution dimeric [Ar*SnH]2 is formed. NMR spectroscopic studies on the kinetics of the DMAP-catalysed reductive elimination of dihydrogen were performed. The activation energy was approximated to be 13.7 kcal mol−1. Solvent dependencies and a kinetic isotope effect KIE of kH/kD = 1.65 in benzene and 2.04 in THF were found and along with DFT calculations support a polar mechanism for this dehydrogenation.
C-bonds in hydro-stannylenylation reactions. The resulting half-sandwich complex Ar*SnCp* was structurally characterized. Moreover, on application of less nucleophilic amine bases, the uncoordinated, in solution dimeric [Ar*SnH]2 is formed. NMR spectroscopic studies on the kinetics of the DMAP-catalysed reductive elimination of dihydrogen were performed. The activation energy was approximated to be 13.7 kcal mol−1. Solvent dependencies and a kinetic isotope effect KIE of kH/kD = 1.65 in benzene and 2.04 in THF were found and along with DFT calculations support a polar mechanism for this dehydrogenation.

 Please wait while we load your content...
Please wait while we load your content...