3D electrogenerated chemiluminescence: from surface-confined reactions to bulk emission
Abstract
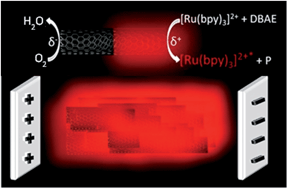
Among luminescence techniques, electrogenerated chemiluminescence (ECL) provides a unique level of manipulation of the luminescent process by controlling the electrochemical trigger. Despite its attractiveness, ECL is by essence a 2D process where light emission is strictly confined to the electrode surface. To overcome this intrinsic limitation, we added a new spatial dimension to the ECL process by generating 3D ECL at the level of millions of micro-emitters dispersed in solution. Each single object is addressed remotely by bipolar electrochemistry and they generate collectively the luminescence in the bulk. Therefore, the entire volume of the solution produces light. To illustrate the generality of this concept, we extended it to a suspension of multi-walled carbon nanotubes where each one acts as an individual ECL nano-emitter. This approach enables a change of paradigm by switching from a surface-limited process to 3D electrogenerated light emission.

 Please wait while we load your content...
Please wait while we load your content...