pH-Controlled selection between one of three guests from a mixture using a coordination cage host
Abstract
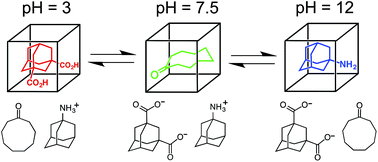
We demonstrate the use of a simple pH swing to control the selection of one of three different guests from aqueous solution by a coordination cage host. Switching between different guests is based on the fact that neutral organic guests bind strongly in the cage due to the hydrophobic effect, but for acidic or basic guests, the charged (protonated or deprotonated) forms are hydrophilic and do not bind. The guests used are adamantane-1,3-dicarboxylic acid (H2A) which binds at low pH when it is neutral but not when it is deprotonated; 1-amino-adamantane (B) which binds at high pH when it is neutral but not when it is protonated; and cyclononanone (C) whose binding is not pH dependent and is therefore the default guest at neutral pH. Thus an increase in pH can reversibly switch the host between the three different bound states cage·H2A (at low pH), cage·C (at medium pH), and cage·B (at high pH) in succession.

 Please wait while we load your content...
Please wait while we load your content...