Access to enantioenriched 2,3- and 2,5-dihydrofurans with a fully substituted C2 stereocenter by Pd-catalyzed asymmetric intermolecular Heck reaction†
Abstract
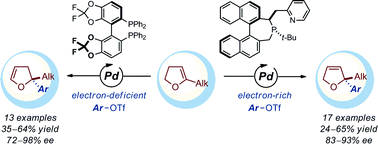
A palladium-catalyzed intermolecular asymmetric Heck reaction with dihydrofurans with a trisubstituted double bond is reported. The use of two different chiral ligands provides access to valuable 2,3- and 2,5-dihydrofurans with a fully substituted C2 stereocenter with high levels of regio- and enantiocontrol.

 Please wait while we load your content...
Please wait while we load your content...