III-defined concepts in chemistry: rigid force constants vs. compliance constants as bond strength descriptors for the triple bond in diboryne
Abstract
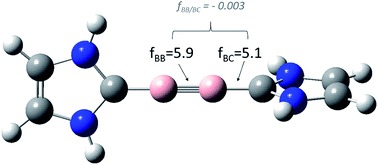
In a recent publication, the interpretation of Braunschweig's diboryne NHC–BB–NHC as a true triple bond is questioned. The analysis by Köppe and Schnöckel is based, inter alia, on the calculation of rigid coupling force constants. Nevertheless, since it is known for a long time that the use of rigid force constants as bond strength descriptors is by no means straightforward, we recomputed the rigid force constants for a model diboryne, applying different coordinate systems and compared the values with the relaxed force constants (generalized compliance constants, GCC). In contrast with the results by Schnöckel and Köppe, the true coupling between the boron–boron bond and the boron–carbon bond, that is, after the elimination of all numerical artifacts, is negligible (fBB/BC = −0.003).

 Please wait while we load your content...
Please wait while we load your content...