Diastereodivergent organocatalysis for the asymmetric synthesis of chiral annulated furans†
Abstract
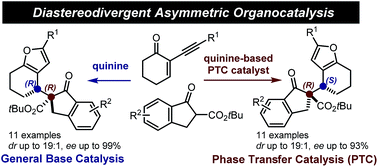
Disclosed herein is a stereoselective method for the synthesis of 2,3-furan fused carbocycles bearing adjacent quaternary and tertiary carbon stereocenters. The chemistry is based on an asymmetric addition of β-ketoesters to 2-(1-alkynyl)-2-alkene-1-ones catalysed by natural cinchona alkaloids followed by a silver-catalysed intramolecular cycloisomerisation. By exploiting the distinct catalysis modes of quinine, which can act either as a general base or, upon opportune modifications, as a phase transfer catalyst, a complete switch of the enforced sense of diastereoinduction is achieved. The stereodivergent systems enable access to the full matrix of all possible stereoisomeric products.


 Please wait while we load your content...
Please wait while we load your content...