Operando X-ray absorption and EPR evidence for a single electron redox process in copper catalysis†
Abstract
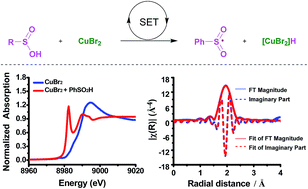
An unprecedented single electron redox process in copper catalysis is confirmed using operando X-ray absorption and EPR spectroscopies. The oxidation state of the copper species in the interaction between Cu(II) and a sulfinic acid at room temperature, and the accurate characterization of the formed Cu(I) are clearly shown using operando X-ray absorption and EPR evidence. Further investigation of anion effects on Cu(II) discloses that bromine ions can dramatically increase the rate of the redox process. Moreover, it is proven that the sulfinic acids are converted into sulfonyl radicals, which can be trapped by 2-arylacrylic acids and various valuable β-keto sulfones are synthesized with good to excellent yields under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...