Insights into the structure–photoreactivity relationships in well-defined perovskite ferroelectric KNbO3 nanowires†
Abstract
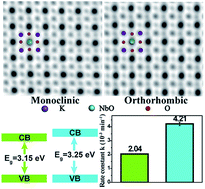
Structure–function correlations are a central theme in heterogeneous (photo)catalysis. In this study, the geometric and electronic structure of perovskite ferroelectric KNbO3 nanowires with respective orthorhombic and monoclinic polymorphs have been systematically addressed. By virtue of aberration-corrected scanning transmission electron microscopy, we directly visualize surface photocatalytic active sites, measure local atomic displacements at an accuracy of several picometers, and quantify ferroelectric polarization combined with first-principles calculations. The photoreactivity of the as-prepared KNbO3 nanowires is assessed toward aqueous rhodamine B degradation under UV light. A synergy between the ferroelectric polarization and electronic structure in photoreactivity enhancement is uncovered, which accounts for the prominent reactivity order: orthorhombic > monoclinic. Additionally, by identifying new photocatalytic products, rhodamine B degradation pathways involving N-deethylation and conjugated structure cleavage are proposed. Our findings not only provide new insights into the structure–photoreactivity relationships in perovskite ferroelectric photocatalysts, but also have broad implications in perovskite-based water splitting and photovoltaics, among others.

 Please wait while we load your content...
Please wait while we load your content...