Hydration of guanidinium depends on its local environment†
Abstract
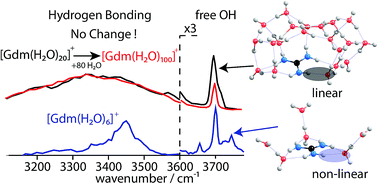
Hydration of gaseous guanidinium (Gdm+) with up to 100 water molecules attached was investigated using infrared photodissociation spectroscopy in the hydrogen stretch region between 2900 and 3800 cm−1. Comparisons to IR spectra of low-energy computed structures indicate that at small cluster size, water interacts strongly with Gdm+ with three inner shell water molecules each accepting two hydrogen bonds from adjacent NH2 groups in Gdm+. Comparisons to results for tetramethylammonium (TMA+) and Na+ enable structural information for larger clusters to be obtained. The similarity in the bonded OH region for Gdm(H2O)20+vs. Gdm(H2O)100+ and the similarity in the bonded OH regions between Gdm+ and TMA+ but not Na+ for clusters with <50 water molecules indicate that Gdm+ does not significantly affect the hydrogen-bonding network of water molecules at large size. These results indicate that the hydration around Gdm+ changes for clusters with more than about eight water molecules to one in which inner shell water molecules only accept a single H-bond from Gdm+. More effective H-bonding drives this change in inner-shell water molecule binding to other water molecules. These results show that hydration of Gdm+ depends on its local environment, and that Gdm+ will interact with water even more strongly in an environment where water is partially excluded, such as the surface of a protein. This enhanced hydration in a limited solvation environment may provide new insights into the effectiveness of Gdm+ as a protein denaturant.

 Please wait while we load your content...
Please wait while we load your content...