Et3B-mediated two- and three-component coupling reactions via radical decarbonylation of α-alkoxyacyl tellurides: single-step construction of densely oxygenated carboskeletons†
Abstract
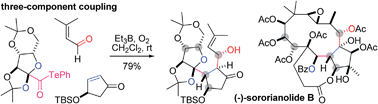
The single-step construction of various densely oxygenated carboskeletons was achieved by radical-based two- and three-component coupling reactions of sugar derivatives, without the need for light or heat. Et3B/O2-mediated decarbonylation readily converted α-alkoxyacyl tellurides to α-alkoxy carbon radicals, which intermolecularly added to glyoxylic oxime ether or enones to provide the two-component adducts. Furthermore, the three-component adducts were produced by an intermolecular aldol reaction between the aldehyde and the boron enolates generated by capture of the two-component radical intermediates by Et3B. This powerful coupling method serves as a novel strategy for the convergent synthesis of polyol natural products.

 Please wait while we load your content...
Please wait while we load your content...