Tuning fluorocarbon adsorption in new isoreticular porous coordination frameworks for heat transformation applications†
Abstract
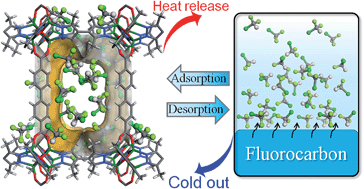
Adsorption heat transformation is one of the most energy-efficient technologies, which relies much on the type and performance of the adsorbent–adsorbate pair. Here, we report adsorption behaviors of a typical fluorocarbon R22 (CHClF2) in a new series of isoreticular porous coordination polymers [Zn4O(bpz)2(ldc)], in which the typical Zn4O clusters are connected by hydrophobic 3,3′,5,5′-tetramethyl-4,4′-bipyrazolate (bpz2−) and different linear dicarboxylates (ldc2−) to form non-interpenetrated pcu networks with variable pore sizes, shapes, and volumes. Fluorocarbon sorption measurements of these materials revealed high R22 uptakes of 0.73–0.97 g g−1 (0.62–0.65 g cm−3) at 298 K and 1 bar and working capacities of 0.41–0.72 g g−1 (0.35–0.47 g cm−3) between 273 and 313 K at about 0.13, 0.11 and 0.52 bar, respectively, as well as very large diffusion coefficients of 5.1–7.3 × 10−7 cm2 s−1. Noteworthily, the R22 sorption performance can be dramatically improved by subtle modification of the pore size and shape, demonstrating porous coordination polymer–fluorocarbon as a promising adsorbent–adsorbate pair for heat transformation applications.
- This article is part of the themed collection: Global Energy Challenges: Materials and Nanotechnology

 Please wait while we load your content...
Please wait while we load your content...