Phenalenyl-fused porphyrins with different ground states†
Abstract
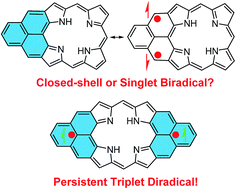
Materials based on biradicals/biradicaloids have potential applications for organic electronics, photonics and spintronics. In this work, we demonstrated that hybridization of porphyrin and polycyclic aromatic hydrocarbon could lead to a new type of stable biradicals/biradicaloids with tunable ground state and physical property. Mono- and bis-phenalenyl fused porphyrins 1 and 2 were synthesized via an intramolecular Friedel–Crafts alkylation-followed-by oxidative dehydrogenation strategy. Our detailed experimental and theoretical studies revealed that 1 has a closed-shell structure with a small biradical character (y = 0.06 by DFT calculation) in the ground state, while 2 exists as a persistent triplet biradical at room temperature under inert atmosphere. Compound 1 underwent hydrogen abstraction from solvent during the crystal growing process while compound 2 was easily oxidized in air to give two dioxo-porphyrin isomers 11a/11b, which can be correlated to their unique biradical character and spin distribution. The physical properties of 1 and 2, their dihydro/tetrahydro-precursors 7/10, and the dioxo-compounds 11a/11b were investigated and compared.

 Please wait while we load your content...
Please wait while we load your content...