Bodipy–C60 triple hydrogen bonding assemblies as heavy atom-free triplet photosensitizers: preparation and study of the singlet/triplet energy transfer†
Abstract
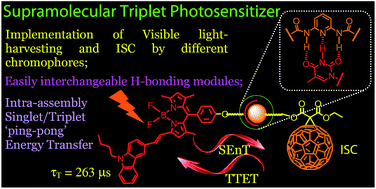
Supramolecular triplet photosensitizers based on hydrogen bonding-mediated molecular assemblies were prepared. Three thymine-containing visible light-harvesting Bodipy derivatives (B-1, B-2 and B-3, which show absorption at 505 nm, 630 nm and 593 nm, respectively) were used as H-bonding modules, and 1,6-diaminopyridine-appended C60 was used as the complementary hydrogen bonding module (C-1), in which the C60 part acts as a spin converter for triplet formation. Visible light-harvesting antennae with methylated thymine were prepared as references (B-1-Me, B-2-Me and B-3-Me), which are unable to form strong H-bonds with C-1. Triple H-bonds are formed between each Bodipy antenna (B-1, B-2 and B-3) and the C60 module (C-1). The photophysical properties of the H-bonding assemblies and the reference non-hydrogen bond-forming mixtures were studied using steady state UV/vis absorption spectroscopy, fluorescence emission spectroscopy, electrochemical characterization, and nanosecond transient absorption spectroscopy. Singlet energy transfer from the Bodipy antenna to the C60 module was confirmed by fluorescence quenching studies. The intersystem crossing of the latter produced the triplet excited state. The nanosecond transient absorption spectroscopy showed that the triplet state is either localized on the C60 module (for assembly B-1·C-1), or on the styryl-Bodipy antenna (for assemblies B-2·C-1 and B-3·C-1). Intra-assembly forward–backward (ping-pong) singlet/triplet energy transfer was proposed. In contrast to the H-bonding assemblies, slow triplet energy transfer was observed for the non-hydrogen bonding mixtures. As a proof of concept, these supramolecular assemblies were used as triplet photosensitizers for triplet–triplet annihilation upconversion.

 Please wait while we load your content...
Please wait while we load your content...