The gold(i)⋯lead(ii) interaction: a relativistic connection†
Abstract
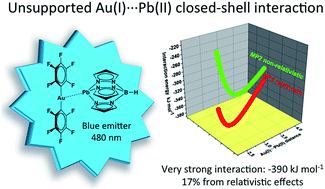
The crystal structure of complex [Pb{HB(pz)3}Au(C6Cl5)2] 1 displays an unsupported Au(I)⋯Pb(II) interaction. This complex emits at 480 nm in the solid state due to an aurate(I) to lead(II) charge transfer, in which the existence of a metallophilic interaction is a pre-requisite. Ab initio calculations show a very strong Au(I)⋯Pb(II) closed-shell interaction of −390 kJ mol−1, which has an ionic plus a dispersive (van der Waals) nature strengthened by large relativistic effects (>17%).

 Please wait while we load your content...
Please wait while we load your content...