A novel copper-chelating strategy for fluorescent proteins to image dynamic copper fluctuations on live cell surfaces†
Abstract
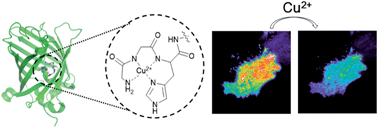
Copper is indispensable in most aerobic organisms although it is toxic if unregulated as illustrated in many neurodegenerative diseases. To elucidate the mechanisms underlying copper release from cells, a membrane-targeting reporter which can compete with extracellular copper-binding molecules is highly desirable. However, engineering a reporter protein to provide both high sensitivity and selectivity for copper(II) has been challenging, likely due to a lack of proper copper(II)-chelating strategies within proteins. Here, we report a new genetically encoded fluorescent copper(II) reporter by employing a copper-binding tripeptide derived from human serum albumin (HSA), which is one of the major copper-binding proteins in extracellular environments. Optimized insertion of the tripeptide into the green fluorescent protein leads to rapid fluorescence quenching (up to >85% change) upon copper-binding, while other metal ions have no effect. Furthermore, the high binding affinity of the reporter enables reliable copper detection even in the presence of competing biomolecules such as HSA and amyloid beta peptides. We also demonstrate that our reporter proteins can be used to visualize dynamic copper fluctuations on living HeLa cell surfaces.

 Please wait while we load your content...
Please wait while we load your content...