Photoinduced dynamics of a cyanine dye: parallel pathways of non-radiative deactivation involving multiple excited-state twisted transients
Abstract
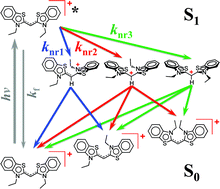
Cyanine dyes are broadly used for fluorescence imaging and other photonic applications. 3,3′-Diethylthiacyanine (THIA) is a cyanine dye composed of two identical aromatic heterocyclic moieties linked with a single methine, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) . The torsional degrees of freedom around the methine bonds provide routes for non-radiative decay, responsible for the inherently low fluorescence quantum yields. Using transient absorption spectroscopy, we determined that upon photoexcitation, the excited state relaxes along two parallel pathways producing three excited-state transients that undergo internal conversion to the ground state. The media viscosity impedes the molecular modes of ring rotation and preferentially affects one of the pathways of non-radiative decay, exerting a dominant effect on the emission properties of THIA. Concurrently, the polarity affects the energy of the transients involved in the decay pathways and further modulates the kinetics of non-radiative deactivation.
. The torsional degrees of freedom around the methine bonds provide routes for non-radiative decay, responsible for the inherently low fluorescence quantum yields. Using transient absorption spectroscopy, we determined that upon photoexcitation, the excited state relaxes along two parallel pathways producing three excited-state transients that undergo internal conversion to the ground state. The media viscosity impedes the molecular modes of ring rotation and preferentially affects one of the pathways of non-radiative decay, exerting a dominant effect on the emission properties of THIA. Concurrently, the polarity affects the energy of the transients involved in the decay pathways and further modulates the kinetics of non-radiative deactivation.

 Please wait while we load your content...
Please wait while we load your content...