Probing the second dehydrogenation step in ammonia-borane dehydrocoupling: characterization and reactivity of the key intermediate, B-(cyclotriborazanyl)amine-borane†
Abstract
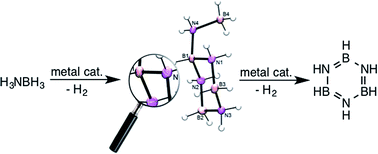
While thermolysis of ammonia-borane (AB) affords a mixture of aminoborane- and iminoborane oligomers, the most selective metal-based catalysts afford exclusively cyclic iminoborane trimer (borazine) and its B–N cross-linked oligomers (polyborazylene). This catalysed dehydrogenation sequence proceeds through a branched cyclic aminoborane oligomer assigned previously as trimeric B-(cyclodiborazanyl)amine-borane (BCDB). Herein we utilize multinuclear NMR spectroscopy and X-ray crystallography to show instead that this key intermediate is actually tetrameric B-(cyclotriborazanyl)amine-borane (BCTB) and a method is presented for its selective synthesis from AB. The reactivity of BCTB upon thermal treatment as well as catalytic dehydrogenation is studied and discussed with regard to facilitating the second dehydrogenation step in AB dehydrocoupling.

 Please wait while we load your content...
Please wait while we load your content...